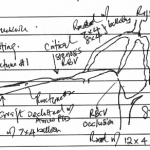
- Line diagram illustrating the findings of and interventions.
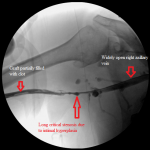
- Right arm venogram: long smooth brachial vein stenosis distal to the graft.
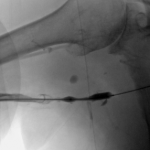
- Balloon dilation of critical venous stenosis distal to the AV graft.
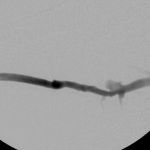
- Right arm venogram post intervention: complete elimination of the stenosis.
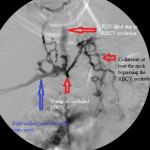
- Central venogram. Note numerous collaterals bypassing the occluded RBCV.
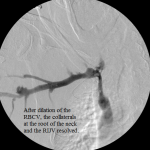
- Central venogram post-dilation of the RBCV: the venous collaterals are resolved.
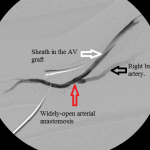
- Angiogram of the arterial anastomosis: there is no stenosis at the arterial anastomosis.
These images illustrate managing peripheral and central venous stenoses associated with a thrombosed hemodialysis access.
They belong to a patient whose graft failed the day after his last hemodialysis. The line diagram is a composite illustration of the anatomy of the graft, the deep veins of the upper right arm, the central veins, observations I made during the intervention, and the materials I employed in the treatment.
The graft is a straight polytetrafluoroethylene graft anastomosed to the distal right brachial artery and the mid right brachial vein. It had neither pulse nor thrill on clinical examination, suggesting it was thrombosed. There were multiple dilated veins on the patient’s upper right chest indicating central venous stenosis.
I established access into the upstream segment of the graft, close to the arterial anastomosis, pointing towards the patient’s head, and centrally advanced a 4 French angled catheter over a stiff glide wire towards the superior vena cava.
I ran into a resistance in the mid right arm that I overcame and an obstruction behind the head of the right clavicle that stopped me in my tracks. I obtained pullback venogram that confirmed these to be due to a long critical stenosis of the mid right brachial vein, immediately distal to the anastomosis of the graft to the vein, and total chronic occlusion of the right brachiocephalic vein, respectively. The latter was associated with numerous ipsilateral and contralateral venous collaterals at the root of the neck as well as refluxing of radiocontrast up the right internal jugular vein.
I mechanically thrombectomized the graft with the Arrow-Trerrotola PTD device inserted through the first access and a second access I established in the distal end of the graft, pointing towards the patient’s hand.
Then I dilated the brachial venous stenosis and probed through and dilated the right brachiocephalic vein. The brachial venous dilation proved optimal, but there was residual stenosis of the RBCV that I resisted stenting at the same setting.
With a balloon inflated at the mid brachial stenosis, I injected radiocontrast through the sheath in the proximal end of the graft, forcing contrast-laden blood to flow back across the arterial anastomosis. This revealed a focal juxtanastomotic stenosis, but not abnormalities of the arterial limb of the circuit. I dilated this second stenosis with optimal outcome.
Though I considered the RBCV disease a threat to the access, I chose to observe the access over time and delay stenting the stenosis for the following reasons:
- The presence of collaterals associated with it suggested that it was not the immediate cause of the graft failure. The collaterals must have provided sufficient pathways of central venous return that allowed optimal venous flow through the graft and normal intra-graft pressure, critical elements in preventing acute graft thrombosis. The most proximate causes of failure of the graft were the juxta-anastomotic stenosis and the critical stenosis of the mid right brachial vein.
- There was no associated swelling of the right upper extremity after I restored function to the graft nor was there any when the patient returned for follow-up at my clinic, proving that the increased blood flow allowed by the plastied mid brachial vein stenosis and the juxta-anastomotic stenosis had sufficient pathways to return to the right atrium without elevating the intragraft pressure unduly.
- Similarly, the sustained thrill and pulse I established after the interventions on the first day were still present when the patient returned for follow-up.
At the time of this writing there is ongoing debate about treating central venous stenoses in patients with failed AVF or AVG, because their role in the failure of these accesses is unclear. Some accesses, as in this patient, seem to tolerate their presence well, while others do not. It seems to me that central venous stenoses that have well-developed collaterals that drain the upper limb well compensate for their lost utility, while in those with little or no collaterals their resistance to the forward push from the arterial limb of the circuit raises intragraft pressure sufficiently to slow down flow through the access and ultimately force it to fail.