History
A 15-year-old boy saw his pediatrician for painless nontraumatic deformity of his left face and chronic nasal stuffiness. The pediatrician referred him to an ear, nose and throat (ENT) surgeon, who learnt from the boy’s mother that her son lost his ability to smell with his left nostril about 1 year before presenting and that he had had 2 episodes of epistaxis through the same nostril during that time. She had attributed his symptoms to common cold until his speech assumed a disturbing nasal quality and his left face swole 1 month before he presented. The surgeon noted a large mass in his left nasal fossa and biopsied it. Acting on the pathologic result of the biopsy specimen, he obtained maxillofacial cross-sectional imaging for the boy and referred him to an interventional radiology clinic for consultation.
Radiologic findings
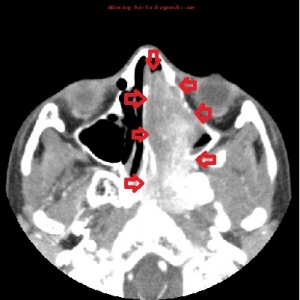
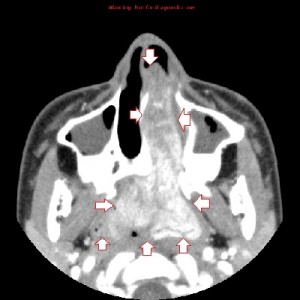
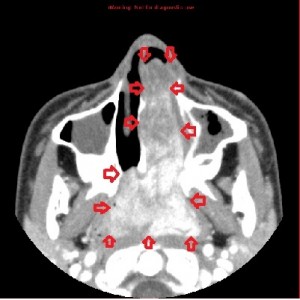
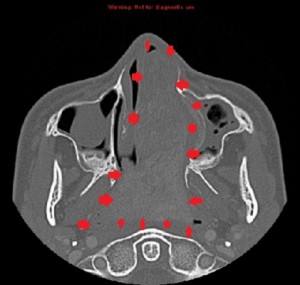
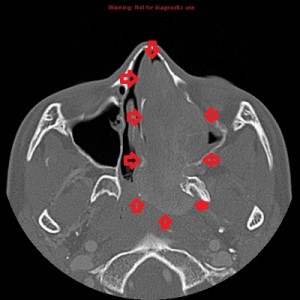
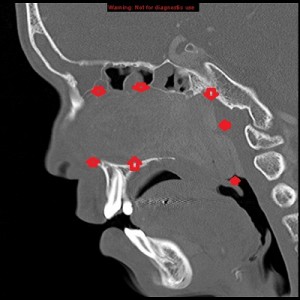
First panel, left to right: Axial contrast-enhanced maxillofacial CT scan in soft-tissue window from superior to inferior, showing a large, avidly enhancing mass in the left nasal fossa, extending posteriorly into the left nasopharnx and crossing into the right nasopharynx behind the nasal septum. Note opacification of the right and left maxillary sinuses, possibly from disturbed drainage.
Second panel, left to right: The same set of images as in the first panel, but in bone window, showing bowing of the medial wall of the left maxillary sinus and opacified maxillary sinuses. There is no bone destruction or invasion.
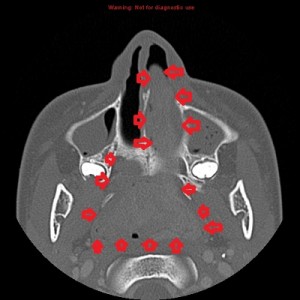
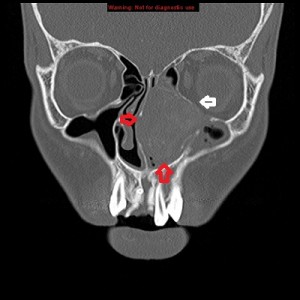
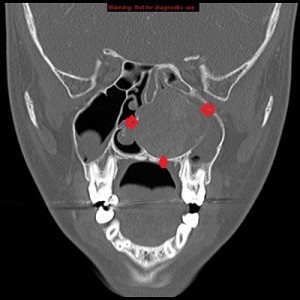
Third panel, left to right: The first 2 images are coronal sections of the face showing the mass-effect of the left nasal mass on other surrounding structure.The third image in this panel is a sagittal paramedian section showing obstruction of the nasopharynx by a soft-tissue mass.
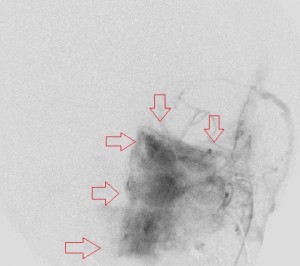
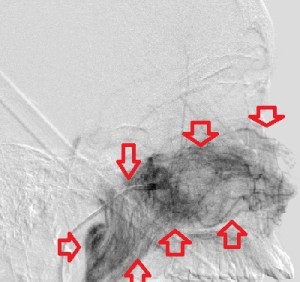
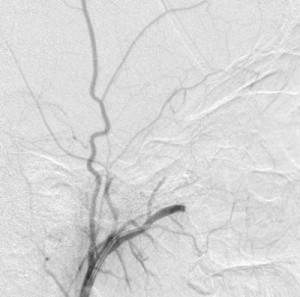
Fourth panel, left to right: Digital subtraction angiogram of the left internal maxillary artery before (1st two images, a frontal and a lateral view of the face) and after embolization (the last image, a lateral view). (For the 2nd and 3rd images, assume the patient’s face is turned to your right). Note the intense blushing of the tumor before embolization emphasizing its vascularity. Following embolization the mass is invisible.
Radiologic diagnosis: Juvenile Nasopharyngeal Angiofibroma.
Pathologic diagnosis: Juvenile Nasopharyngeal Angiofibroma.
Discussion
Juvenile nasopharyngeal angiofibroma (JNA) is a benign locally aggressive hamartoma of the nasopharynx, first described in 1906 by Chaveau and constitutes only 0.05% of head and neck tumors. It occurs almost exclusively in the adolescent and young male population that its occurrence in a female should prompt chromosomal studies and measurement of serum 17-ketosteroids for sex determination. Its usual age range for occurrence is between 10 and 24 years, with a median age at diagnosis of 15 years.
JNA arises from the upper border of the sphenopalatine foramen and, as it grows, may extend into the infratemporal fossa, the sphenoid sinus, the maxillary antrum, the cavernous sinus, the middle cranial fossa , and, rarely, the anterior cranial fossa. Though its exact cause is unknown, the tumor is thought to arise from remnant vascular plexus of the first branchial artery. Because it occurs at a time of facial bone development 50% of patients present with remodelled or damaged bones of the paranasal sinuses, more by expansion than by invasion. The most common bones affected are the maxilla, sphenoid, pterygoid plates, orbital apex and the skull base. In deed, the anterior bowing of the posterior wall of the maxilla at the level of the ptyregoid pillars, the Holman-Miller sign, gives the tumor its most consistent and reliable radiologic feature, although lymphoepithelioma and fibrous dysplasia are two rare nasopharyngeal tumors that have on occasion caused anterior bowing of the posterior maxillary wall.
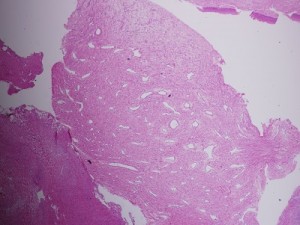
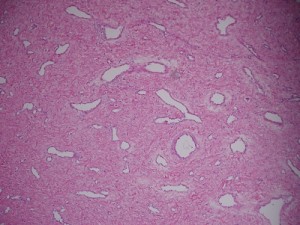
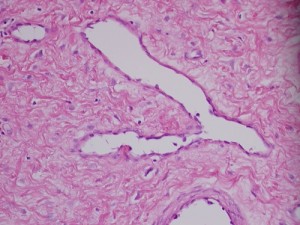
The tumor is composed of spindle-shaped and stellate cells embedded in a collagen matrix and a complex of blood vessels that vary in size from capillaries to large venous channels and have no elastic lamina or elastic fibers; this latter characteristic explains its tendency to easy recurrent bleeding. Thus, severe recurrent epistaxis, occasionally life-threatening, occurs in 63% of patients, second only to unilateral nasal obstruction (91%) as a presenting symptom. Other related symptoms of the disease are nasal discharge, pain, sinusitis, facial deformity, hearing impairment, otitis media, proptosis and diplopia, each reflecting JAN’s mass-effect on its neighbors. These symptoms generally present for 6 months to 1 year before the patient is diagnosed.
The diagnosis of JAN is straight forward: a young male with recurrent epistaxis and nasal obstruction found to have an obstructing nasal or nasopharyngeal mass in the absence of constitutional symptoms (fever, weight loss, lymphadenopathy (lypmhoepithelioma)). Standard imaging includes maxillofacial plain radiographs and contrast-enhanced computerized tomographic scan (CT scan) and magnetic resonance imaging (MRI). The plain radiograph reveals a nasopharyngeal soft-tissue mass associated with anterior bowing of the posterior wall of the maxilla; CT demonstrates the mass and any osseous involvement that may limit resection of the tumor and predict its recurrence; MRI’s superior soft-tissue imaging highlights the extent of the tumor and, with diffusion-weighted sequence helps distinguish JAN from a malignant nasopharyngeal tumor. Furthermore, MRI is useful for detecting tumor recurrence and should generally be obtained within 1 year after tumor resection for baseline information.
Severel treatment options for JNA have been described in the literature, some now obsolete. They include hormonal therapy (the tumor has receptors for many male hormones), radiation, embolization, chemotherapy, open surgical removal, and, in the last 2 decades, endoscopic resection of the mass. Hormonal therapy is largely abandoned for its ineffectiveness. Radiotherapy has been applied as definitive treatment for advanced JAN and has local control rates between 85% and 91%. However, it may be complicated by panhypopituitarism, growth retardation, cataracts, radiation keratopathy, temporal lobe necrosis, and malignant transformation of the tumor, especially with high doses of radiation. It is perhaps excellent in treating primary or recurrent disease in critical areas, where surgical excision of the tumor may be hazardous. Open surgical extirpation was the only method of resecting the disease before the advent of modern novel techniques like endoscopic resection and radiofrequency coblation. There are many approaches to resecting the disease each dependent on the location and extent of the tumor. It is sometimes combined with endoscopic resection. Bland embolization of JNA is ususally offered as a presurgical intervention to limit hemorrhage during surgical removal. The usual arterial source to the tumor is the internal maxillary artery, but it may receive branches from the ophthalmic artery, ascending pharyngeal artery, and the facial artery. Identifying and embolizing these sources reduces the amount of intra-operative blood loss by 50% or more and creates a relatively bloodless operating field that improves tumor resection. When embolization is offered, surgical resection should be done in 2 to 5 days to avoid the development of collateral supply to the tumor. Endoscopic resection of the tumor enjoys certain advantages over traditional open surgery: comesis (no facial scars), shorter hospital stay that translates to lower cost; shorter recuperation; and better or equal recurrence rate. However, there are controversies over what stage of the disease is best treated by this technique. (I have eliminated the staging of JAN in this writing for want of space.)
Recurrence of JAN varies between 13% and 46% depending on the size of the tumor, its extension including osseous penetration, and the surgical approach used to remove it. Most recurrences occur within 12 months and are usually due to incomplete excision of the tumor.
This patient’s course
His laboratory investigation was unremarkable and the tumor was successfully resected endoscopically following presurgical embolization of his terminal left internal maxillary artery; the estimated blood loss was 350 mL. He did well following surgery but defaulted on followups. This month celebrates his first year of the resection of the tumor.
Acknowledgement
The pathology slides are by the kind auspices of Dr. Shanching Ying, Pathologist and Director of Microbiology Lab, Department of Pathology, Mount Sinai Medical Center, Chicago, Illinois.



